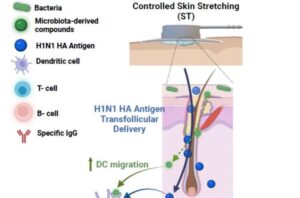
Skin stretching enables needle-free vaccine delivery in mice
Researchers show that stretching the skin stimulates immune cells and increases the skin’s ability to absorb large molecules, including those present in vaccines.

Researchers show that stretching the skin stimulates immune cells and increases the skin’s ability to absorb large molecules, including those present in vaccines.
Developed by Stanford researchers, NeuroString is a hair-thin multichannel biosensor and stimulator with promising potential applications in drug delivery, nerve stimulation, smart fabrics, and more.

Tulane University researchers have developed an enhanced CRISPR-based tuberculosis test that works with a simple tongue swab, a potential breakthrough that could allow easier, community-based screenings for the world’s deadliest infectious disease.
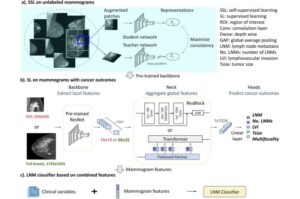
A project at Lund University in Sweden has trained an AI model to identify breast cancer patients who could be spared from axillary surgery. The model analyzes previously unutilized information in mammograms and pinpoints with high accuracy the individual risk of metastasis in the armpit.

A powder based on morin, a natural compound extracted from plants such as guava leaves, apple and fig peels, certain teas, and almonds, has shown antimicrobial, anti-inflammatory, and antioxidant effects against bacteria that cause periodontal disease.

Researchers have developed a generative AI model that uses large-scale health records to estimate how human health may change over time. It can forecast the risk and timing of over 1,000 diseases and predict health outcomes over a decade in advance.
A newly developed molecule brings together two powerful immunotherapy strategies in one treatment. Researchers at the University of Basel and University Hospital Basel, Switzerland, have demonstrated that this fusion protein can both block the “do not attack” signal used by cancer cells and selectively activate tumor-fighting immune cells.
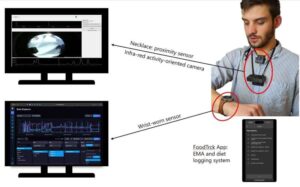
What if your smart watch could sense when you’re about to raid the fridge, and gently steer you toward a healthier choice instead?

This manufacturing site in Richmond, Virginia, is the first of four projects that Eli Lilly plans to reveal this year as part of a $27 billion U.S. investment announced earlier this year.
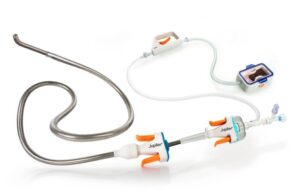
Jupiter Endovascular announced today that its Vertex catheter received FDA 510(k) clearance for the insertion of endovascular devices.