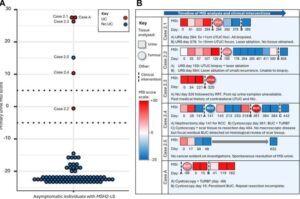
Urine DNA test may spot Lynch syndrome urinary cancers before symptoms
A pioneering genetic test is improving early diagnosis and treatment for people with hereditary cancer caused by a genetic condition. The test, developed with the help of Newcastle University scientists, identifies specific signs in a person’s DNA that are characteristic of cancers linked with Lynch syndrome.