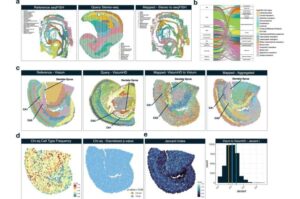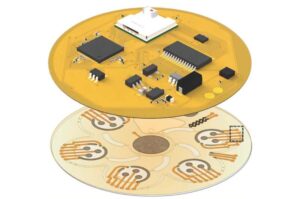
3D-printed scaffold process offers hope for spinal cord injury recovery
For the first time, a research team at the University of Minnesota Twin Cities demonstrated a process that combines 3D printing, stem cell biology, and lab-grown tissues for spinal cord injury recovery.