AI model spots gastric cancer on routine CT scans with high accuracy, outperforming radiologists
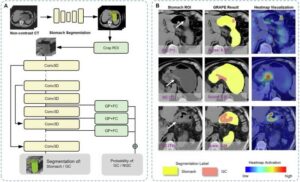
A collaboration of leading Chinese research institutions has developed an artificial intelligence-based method called GRAPE, demonstrating high accuracy in detecting gastric cancer from routine noncontrast CT scans.