Bioengineered skin doubles burn healing speed in preclinical models
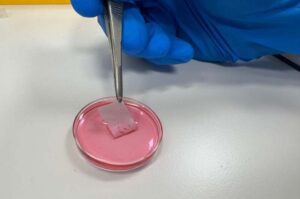
Researchers from Tel Aviv University and Sheba Tel Hashomer Medical Center have developed an innovative bioengineered skin equivalent for grafting in burn victims. The bioengineered skin produced from the patient’s own cells is more stable, robust, and flexible than current treatments, making it easier to handle.