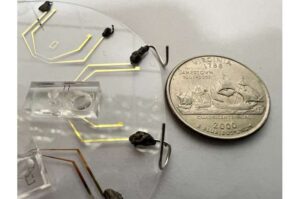
Engineers develop portable device to detect rare mutations
A team led by Rutgers University-New Brunswick engineers has developed a portable device capable of detecting rare genetic mutations from a single drop of blood.

A team led by Rutgers University-New Brunswick engineers has developed a portable device capable of detecting rare genetic mutations from a single drop of blood.
A new study led by Keck Medicine of USC researchers may have uncovered an effective combination therapy for glioblastoma, a brain tumor diagnosis with few available effective treatments. According to the National Brain Tumor Society, the average survival for patients diagnosed with glioblastoma is eight months.
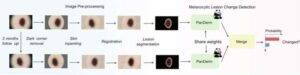
Detection of melanoma and a range of other skin diseases will be faster and more accurate with a new AI-powered tool that analyzes multiple imaging types simultaneously, developed by an international team of researchers led by Monash University.

For the nearly 40 million Americans living with diabetes, an important part of managing the disease is monitoring blood sugar throughout the day and night.
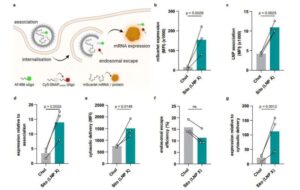
A major bottleneck in curing HIV (human immunodeficiency virus) is that the virus can hide in an inactive form within resting white blood cells, which play a crucial role in coordinating the immune response.

Regulatory Milestone Validates JBA AI’s Clinical-Grade Software for Advanced Diagnostic Support

A Medtronic (NYSE: MDT)+
official announced on social media that the company received CE mark for its VitalFlow system within its Cardiac Surgery portfolio.

This tiny device can shrink dangerous blood clots. It’s called the ‘milli-spinner’ and its invention was partly an accident.
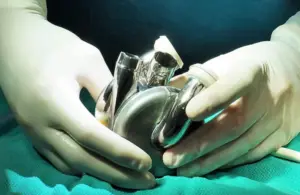
BiVacor announced today that it received FDA breakthrough device designation for its Total Artificial Heart (TAH) system.
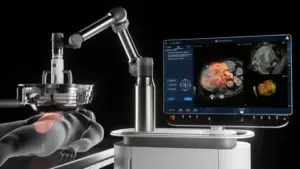
Non-Invasive Histotripsy Technology Authorized Under Unmet Clinical Needs Pathway for Liver Tumor Treatment