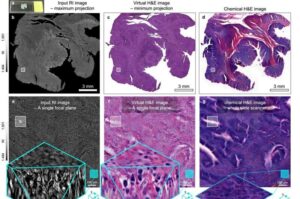
3D virtual staining technology enables non-invasive observation of cancer tissue
A research team led by Professor YongKeun Park of the Department of Physics, in collaboration with Professor Su-Jin Shin’s team at Yonsei University Gangnam Severance Hospital, Professor Tae Hyun Hwang’s team at Mayo Clinic, and Tomocube’s AI research team, has developed an innovative technology capable of vividly displaying the 3D structure of cancer tissues without separate staining.