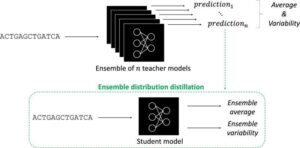
Blood and urine DNA tests may help some bladder cancer patients avoid surgery
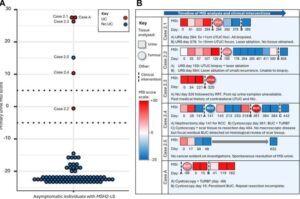
Researchers at the Icahn School of Medicine at Mount Sinai have reported promising findings that may help redefine treatment for patients with muscle-invasive bladder cancer, a potentially aggressive form of the disease traditionally treated with surgical removal of the bladder. The study, published in the Proceedings of the National Academy of Sciences, demonstrates that ultra-sensitive testing of tumor-derived DNA in blood and urine may help identify patients who can safely preserve their bladder without compromising cancer outcomes.