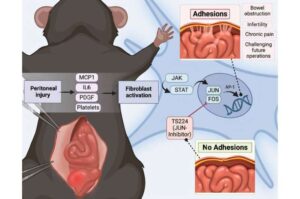
An easy-to-apply gel prevents abdominal adhesions following surgery in animal study
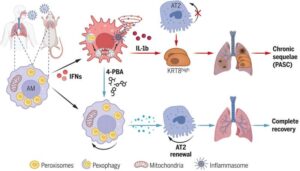
Surgical adhesions—common, sometimes life-threatening complications that arise after open or laparoscopic abdominal surgery—can be prevented in mice and pigs by a gel impregnated with a molecule that blocks a key signaling pathway in the formation of scar tissue.