Philips radiology imaging system wins EU MDR certification
Philips this week announced it received EU MDR certification for remote scanning capabilities on its Radiology Operations Command Center.

Philips this week announced it received EU MDR certification for remote scanning capabilities on its Radiology Operations Command Center.

SANTA BARBARA, Calif., Feb. 24, 2025 /PRNewswire/ — Aptitude Medical Systems, Inc. (Aptitude) today announced it has received Emergency Use Authorization (EUA) from the U.S. Food and Drug Administration (FDA) for its next-gen molecular Metrix® COVID/Flu multiplex test. This innovative test represents a major advancement in accessible molecular diagnostics.
Application covering a wide range of indications is in line with trends in de-escalation of surgery and growth in minimally invasive cryoablation procedures, pointing to strong potential for increasing demand

Medtronic (NYSE: MDT)+
announced today that the FDA approved its BrainSense adaptive deep brain stimulation (aDBS) platform.
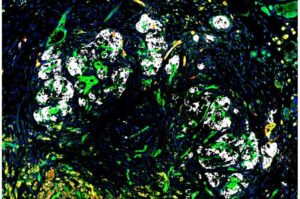
Adding engineered human blood vessel-forming cells to islet transplants boosted the survival of the insulin-producing cells and reversed diabetes in a preclinical study led by Weill Cornell Medicine investigators. The new approach, which requires further development and testing, could someday enable the much wider use of islet transplants to cure diabetes.
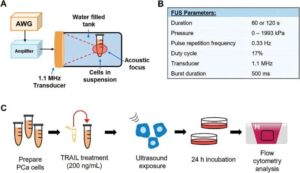
Combining an existing small-molecule protein therapy called tumor necrosis factor related apoptosis-inducing ligand (TRAIL) with focused ultrasound (FUS) can significantly reduce tumor size and burden in prostate cancer models, according to a new study published in Advanced Science by researchers at Rice University and Vanderbilt University.
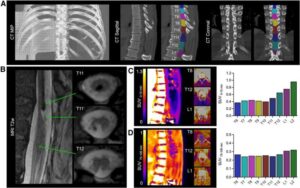
A novel PET technique that visualizes spinal cord injuries provides critical information about which patients may be able to regain mobility, according to new research published in the February issue of The Journal of Nuclear Medicine. By detecting intact nerve connections in the injured spinal cord, a newly developed radiotracer has the potential to help diagnose injuries more precisely, monitor recovery, and evaluate the effectiveness of new therapies in clinical trials.
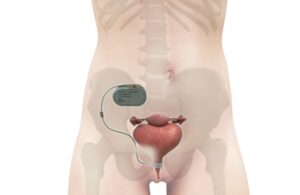
UroMems announced that the entire treatment cohort in the first-of-its-kind study in female patients successfully reached its primary endpoints.
When even the most highly trained surgeons perform procedures on the retina—one of the smallest, most delicate parts of the human body—the stakes are high. Surgeons must account for patients’ breathing, snoring, and eye movements, along with their own involuntary hand tremors, while they work on a layer of cells less than a millimeter thick.
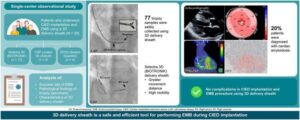
Endomyocardial biopsy (EMB) is a valuable method for diagnosing a range of cardiac conditions, but there is a risk of complications due to its invasive nature. Researchers have now found a way to combine right ventricular (RV) septal EMB, exploiting the benefit of 3D curved conduction system pacing (CSP) sheaths, with subsequent cardiac implantable electronic device (CIED, devices like pacemakers or defibrillators to regulate heart rhythm) implantation, using the same sheath.