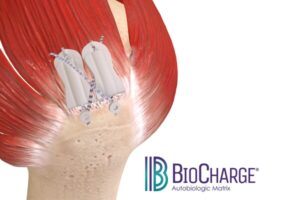
Atreon Orthopedics Announces FDA 510(k) Clearance and Full Market Launch of BioCharge® Autobiologic Matrix for Rotator Cuff Repair
DUBLIN, Ohio, Feb. 20, 2025 /PRNewswire/ — Atreon Orthopedics, LLC, a Columbus based innovator in tissue healing and regenerative technologies, announces the 510(k) clearance from the Food and Drug Administration (FDA) and the full market launch of BioCharge® Autobiologic Matrix, a bioresorbable synthetic implant designed to address biological failure modes in rotator cuff repair while improving repair integrity and long-term patient outcomes.