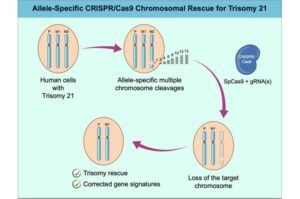
Scientists develop new natural killer cell strategy to target HIV
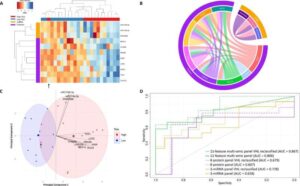
Researchers at The Wistar Institute’s HIV Cure and Viral Diseases Center have successfully identified a new approach using natural killer (NK) cells to target and kill the HIV-positive cells that allow the virus to persist. Wistar scientists have labeled this new approach “NuKES”: Natural Killer Enhancement Strategy.