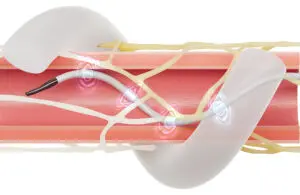
Medtronic achieves renal denervation reimbursement milestone with CMS
Medtronic (NYSE: MDT)+
announced that CMS is opening a national coverage analysis on renal denervation (RDN) procedures for patients with hypertension.

Medtronic (NYSE: MDT)+
announced that CMS is opening a national coverage analysis on renal denervation (RDN) procedures for patients with hypertension.
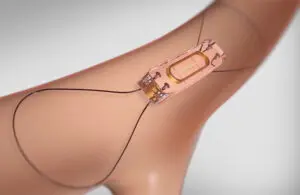
An Abbott (NYSE: ABT)+
official said on social media that the company secured a significant Medicare win for its CardioMEMS platform.

Biobot Surgical announced today that it received CE mark for its Mona Lisa 2.0 surgical robotic platform for urology applications.
TEL AVIV, Israel, Jan. 14, 2025 /PRNewswire/ — BAIBYS™, a pioneering Israeli startup in AI-powered micro-robotics for Artificial Reproductive Technology (ART), today announced it has received the CE mark for its innovative BAIBYS™ system.
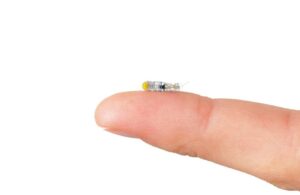
Robeauté announced today that it raised $28 million to support the development of its neurosurgical microrobot technology.

New discovery makes system monitoring contaminants in drinking water 10 times more sensitive

ENGLEWOOD CLIFFS, N.J., Jan. 13, 2025 /PRNewswire/ — BRAIN.Q, is excited to announce the CE Mark for its BQ 2.0 system, designed to reduce disability following ischemic stroke, the number one cause of disability worldwide.

FREMONT, Calif., Jan. 13, 2025 /PRNewswire/ — Q’Apel Medical (Q’Apel), a privately held medical device company focused on revolutionizing neurovascular interventions, today announced it has received CE mark certification for its Armadillo SelectFlex™ Neurovascular Access System. The first-of-its-kind 7F system features the patented SelectFlex™ Technology and is indicated for the introduction of interventional devices into the neurovasculature.
DUBLIN, Calif. and JENA, Germany, Jan. 13, 2025 /PRNewswire/ — ZEISS Medical Technology announced today that the MEL® 90 received approval from the U.S. Food and Drug Administration (FDA), giving the excimer laser technology simultaneous approval for all three major indications, including myopia, hyperopia and mixed astigmatism (a condition where both hyperopic and myopic correction is required).
WOBURN, Mass., Jan. 13, 2025 /PRNewswire/ — Spear Bio Inc., a biotechnology company pioneering next-generation ultra-sensitive immunoassays, today announced that its pTau 217 blood test has been granted Breakthrough Device Designation by the U.S. Food and Drug Administration (FDA). This recognizes the test’s potential to address a critical unmet need for the millions of Americans living with Alzheimer’s disease and not yet diagnosed.