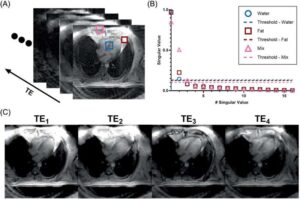
New MRI technique identifies heart disease risk from fat composition
Everyone knows the health risks of carrying too much fat around the waist and hips, but UVA Health scientists are developing a noninvasive way to assess the health risks of unseen fat around the heart.