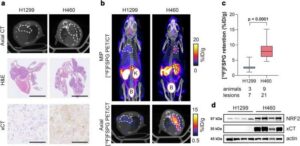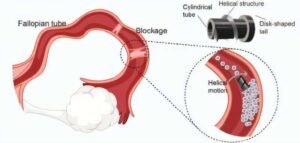
Magnetically driven robotic microscrews offer a new solution for fallopian tube blockages
Infertility affects an estimated 186 million people worldwide, with fallopian tube obstruction contributing to 11–67% of female infertility cases. In AIP Advances researchers at the SIAT Magnetic Soft Microrobots Lab have developed an innovative solution using a magnetically driven robotic microscrew to treat fallopian tube blockages.