Paper-based biosensor offers pain-free diabetes monitoring via sweat
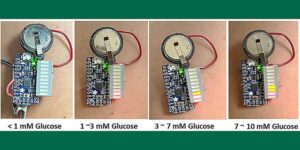
Millions of people with diabetes track their glucose levels daily using finger-stick devices that draw and analyze their blood. But what if they could monitor it with just a sweat sensor?