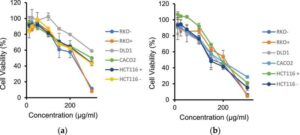
Aromatic plant can fight colorectal cancer, say scientists
Scientists from the University of Sharjah have found that a naturally growing aromatic plant contains ingredients with the ability to treat colorectal cancer.

Scientists from the University of Sharjah have found that a naturally growing aromatic plant contains ingredients with the ability to treat colorectal cancer.

Imperative Care announced today that it has secured FDA 510(k) clearance for its Zoom comprehensive stroke thrombectomy system.

Stereotaxis (NYSE:STXS) said today that it has secured CE mark approval for its Magic robotically-navigated magnetic ablation catheter.
Researchers at the University of Colorado Anschutz Medical Campus have found a promising drug candidate that could help restore vision in individuals with multiple sclerosis (MS) and other neurological conditions that damage neurons.
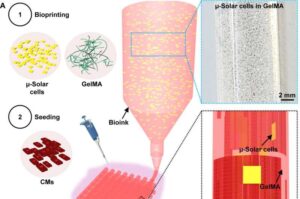
Researchers from Mass General Brigham and collaborating institutions have developed a non-invasive approach to manipulate cardiac tissue activity by using light to stimulate an innovative ink incorporated into bioprinted tissue.
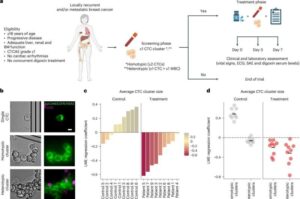
In a study, which has been published in Nature Medicine, a team of researchers from ETH Zurich, the University Hospitals of Basel and Zurich, and the Basel-Land Cantonal Hospital, shows a new, promising approach

The nanoparticle-based vaccine shows promise against many variants of SARS-CoV-2, as well as related sarbecoviruses that could jump to humans.
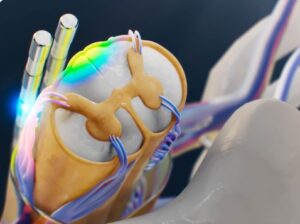
MINNEAPOLIS, Jan. 23, 2025 /PRNewswire/ — Saluda Medical, Inc., a pioneer in the development and commercialization of a novel neuromodulation platform designed to transform the lives of patients with chronic neurological conditions, today announced United States (U.S.) Food & Drug Administration (FDA) approval for its biomarker-based, automated patient programming platform in spinal cord stimulation (SCS), representing a significant advancement for SCS therapy.
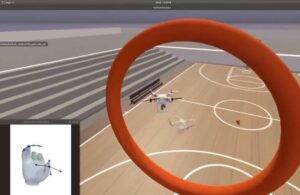
Blackrock Neurotech announced that researchers used its microelectrode arrays to develop a high-performance brain-computer interface (BCI).
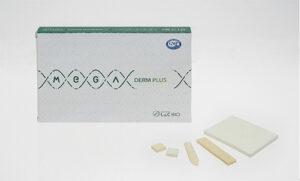
SEONGNAM-SI, South Korea, Jan. 22, 2025 /PRNewswire/ — L&C Bio (CEO Hwan-Chul Lee) has achieved a significant milestone in its global market strategy by becoming the first company worldwide to secure approval from China’s National Medical Products Administration (NMPA) for its acellular dermal matrix, “MegaDerm Plus.”