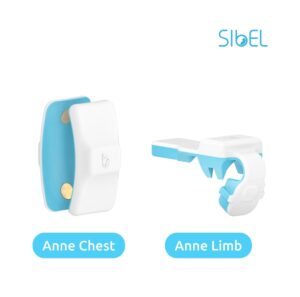
Two New FDA-Clearances for Sibel Health Enables Compatibility of Advanced Wearable Sensors to 3rd Party Software Solutions
CHICAGO, Aug. 22, 2024 /PRNewswire/ — Sibel Health announces two additional FDA-clearances enabling its advanced wearable sensors to operate with compatible third party software applications. In order to democratize vital signs globally, Sibel Health is committed to enabling widespread access to its advanced wearable sensors.