
FDA approves Microbot Medical endovascular surgical robot trial
Microbot Medical (Nasdaq:MBOT) this week announced it received FDA approval to start its human clinical trial for its Liberty Endovascular Robotic Surgical System.

Microbot Medical (Nasdaq:MBOT) this week announced it received FDA approval to start its human clinical trial for its Liberty Endovascular Robotic Surgical System.
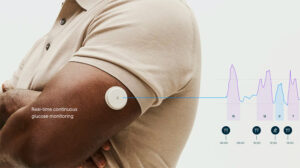
Abbott (NYSE: ABT)+ has secured a 510(k) clearance from the FDA for its over-the-counter Lingo glucose-monitoring biowearable.

SOUTH SAN FRANCISCO, Calif., May 30, 2024 /PRNewswire/ — Enable Biosciences Inc. has been awarded a patent for a cutting-edge method of detecting polyclonal antibodies, marking a significant advancement in diagnostic technologies, particularly in assessing the risk of Type 1 Diabetes (T1D).

The EU Medical Device Regulation CE certification approves that the most common types of skin cancer and a wide range of skin diseases and conditions can be treated in less than 90 seconds.

Spineart recently announced it received FDA 510(k) clearance for its Scarlet AC-Ti secured anterior cervical cage.

Terumo Cardiovascular today announced FDA 510(k) clearance of its next-generation CDI OneView monitoring system.

BUENA, N.J., May 29, 2024 /PRNewswire/ — COMAR, a leading provider of innovative medical devices and packaging solutions, is thrilled to announce a significant achievement. After rigorous preparation, they successfully obtained their CE certificate under the latest EU Medical Device Regulations (EU MDR 2017/745).
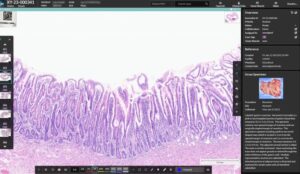
HALO AP Dx brings digital primary diagnosis to anatomic pathology labs in the US.

Canary Medical announced today that it received FDA breakthrough device designation for its Canturio lumbar cartridge.
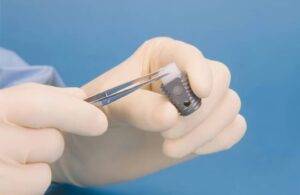
Medtronic (NYSE: MDT)+
announced today that the FDA granted breakthrough device designation for its Infuse bone graft.