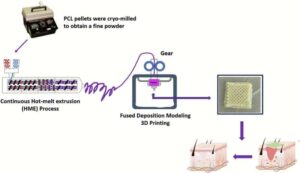
Team develops 3D-printed bandage to help heal chronic wounds
A team of University of Mississippi researchers is developing a way to use 3D-printed medicated patches to help close persistent sores and ulcers

A team of University of Mississippi researchers is developing a way to use 3D-printed medicated patches to help close persistent sores and ulcers
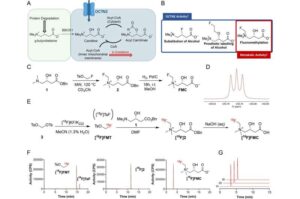
A new King’s College London study introduces a brand-new imaging tracer that looks at how tumors use fats to fuel their growth
Oregon Health & Science University researchers have found that certain nerves that play an integral role in the body’s “fight or flight” stress response can support pancreatic tumor growth.
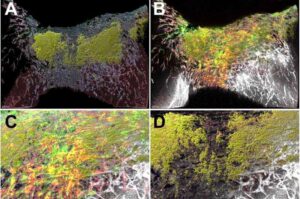
Researchers from the Centenary Institute and the University of Technology Sydney (UTS) have developed a human heart cell model demonstrating that the virus that causes COVID-19 (SARS-CoV-2) can directly infect heart tissue, providing new insight into why some people experience serious heart complications during and after infection.

A new, fully customizable 3D printed socket design is set to transform the prosthetics industry.
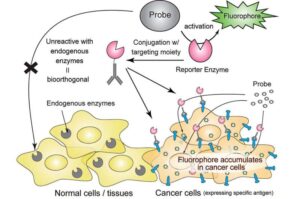
In tests on mice, we delivered a special enzyme to tumors and used a fluorescence probe that only turns on when that enzyme is present,

A new study by researchers at Wake Forest University School of Medicine has identified a promising strategy to treat brain metastases, one of the most challenging and deadly complications of lung cancer.

A wearable device that alerts people with food allergies before a reaction begins has the potential to reduce life-threatening anaphylaxis and transform allergy management from reactive to preventive care.
Scientists at Duke-NUS Medical School have identified a molecular “switch” that determines whether pancreatic cancer cells resist chemotherapy or respond to it—a finding that could help convert some of the most treatment-resistant tumors into forms that are more manageable with existing drugs.

Researchers at The Jackson Laboratory (JAX), in collaboration with the Massachusetts Institute of Technology (MIT), have developed the first bandage-like microneedle patch that can sample the body’s immune responses painlessly from the skin.