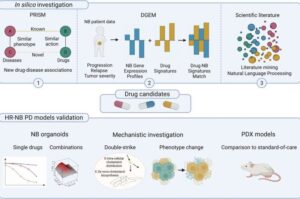
Machine learning identifies statin and phenothiazine combo for neuroblastoma treatment
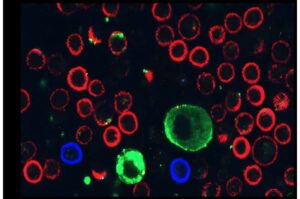
Researchers at Lund University in Sweden have identified a combination of statins and phenothiazines that is particularly promising in the treatment of the aggressive form of neuroblastoma.