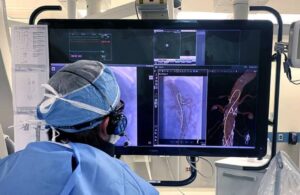
Dr. Carlos Timaran, a vascular surgeon, marked the launch with the first use of the guidewire on a patient. It features the company’s breakthrough Fiber Optic RealShape (FORS) technology. Timaran performed a complex aortic aneurism repair operation.
Philips said in a news release that introducing the new, FORS-enabled wire gives U.S. clinicians a way to visualize a broader range of catheters. It expands the usage of the technology to patients in the U.S. treated in centers equipped with the company’s LumiGuide technology.
Timaran called the new guidewire a “significant leap forward in endovascular surgery.” He added that it has potential to “revolutionize how we approach minimally invasive vascular interventions.”