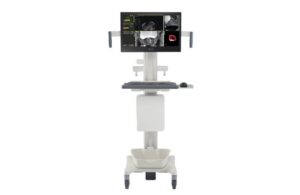
The regulatory nod marks the latest advancement in image-guided navigation for prostate cancer care from the Amsterdam-based medtech giant. UroNav now features a new, advanced annotation workflow that supports clinicians during focal therapy procedures. It helps to deliver more precise, minimally invasive care.
As an image fusion system, UroNav integrates pre-procedural imaging (like MRI) with real-time, intra-procedural ultrasound imaging. It enhances the precision and accuracy of therapeutic procedures with a comprehensive view of the targeted area, Philips says.
According to Philips, a more targeted approach enables a more informed treatment selection with better, more precise care. The new advanced annotation workflow works in tandem with DynaCAD Urology to support focal therapy planning, delivery and review.