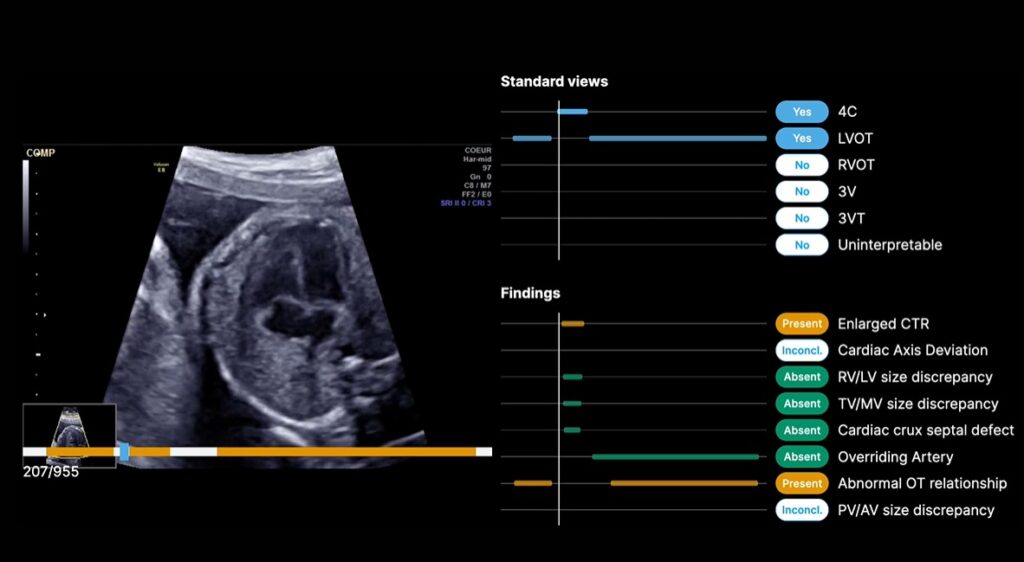
The obstetrics and pediatric cardiology medical device company, based in Paris, announced yesterday that it received the new clearance for the addition of a cart-side tablet that clinicians can use during fetal heart ultrasound evaluations. In November 2024, the FDA granted the company its initial 501(k) clearance for its AI software.
The AI platform is designed to integrate into existing workflows and flag structural markers associated with CHDs. Up to 70% of CHDs go undetected by clinicians conducting routine prenatal ultrasound exams, according to BrightHeart. And the company hopes to change that with this digital screening tool.
The BrightHeart tablet was piloted in clinics, where it received positive feedback.