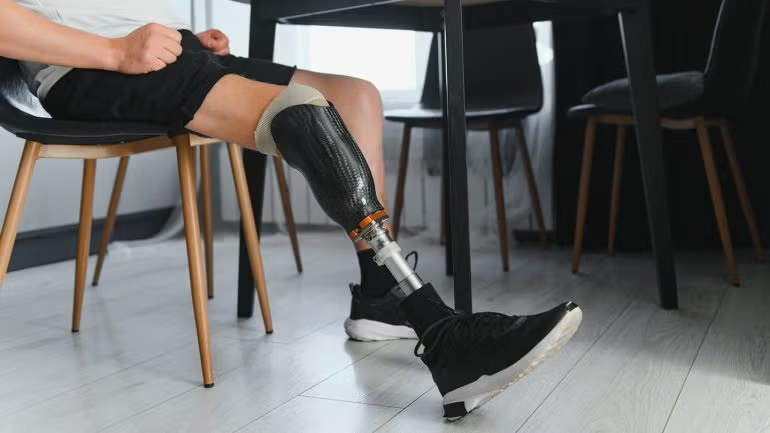
The US Food and Drug Administration (FDA) has granted investigational device exemption (IDE) approval for Renerva to initiate the first human clinical trial of the Renerva PNM-CAP nerve capping device.
The trial will take place at The Ohio State University Wexner Medical Center, US, with Amy Moore serving as principal investigator.
The device is intended to cap nerves following transection, a frequent occurrence in amputations, to prevent the formation of neuromas.