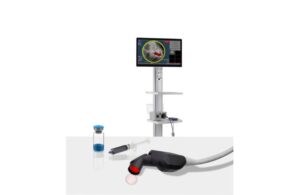
LumiSight, an optical imaging agent, works in combination with the Lumicell direct visualization system (DVS) in breast cancer imaging. The combination product — a fluorescence-guided surgical imaging tool — detects residual cancer in real-time during a lumpectomy.
Lumicell’s system enables real-time assessments of the breast cavity to remove residual cancer tissue. Such tissue would otherwise have been missed, according to Dr. Shelly Hwang. In a news release, Hwang, leader of Duke University’s Breast Oncology Program, said it’s “unlike any other surgical tool available” today.
The FDA’s Medical Imaging Drugs Advisory Committee (MIDAC) voted 16-2 in favor of LumiSight. One voter abstained.