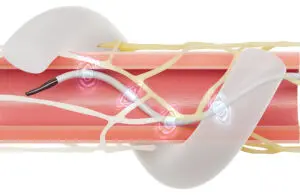
Symplicity Spyral is the first high-blood-pressure-treating renal denervation (RDN) system to be approved by China’s NMPA. The company said it intends to go through the provincial registration process and expects sales of the RDN system in China to be modest in the short term.
The approval comes nearly six months after Medtronic won FDA approval for Symplicity Spyral, becoming the second company in the U.S. to have premarket approval for an RDN system. In March of this year, it received Health Canada licensing.