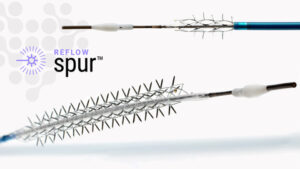
Reflow Medical Receives CE Mark for Bare Temporary Spur Stent System for Treating de novo or Restenotic Below-the-Knee (BTK) Lesions
Reflow Medical, Inc., a developer of innovative medical devices focused on cardiovascular disease, announces it has received CE (Conformité Européenne) Mark certification in the European Union for the Bare Temporary Spur Stent System.