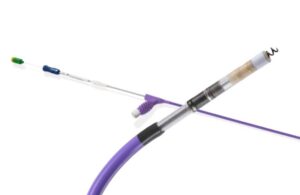
FDA approves expanded label for Boston Scientific Ingevity+ pacing leads
Boston Scientific (NYSE: BSX)+
announced today that it received FDA approval to expand the indication for its current-generation Ingevity+ pacing leads.

Boston Scientific (NYSE: BSX)+
announced today that it received FDA approval to expand the indication for its current-generation Ingevity+ pacing leads.
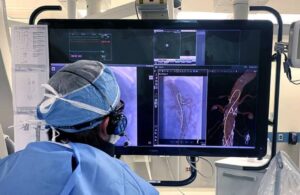
Philips (NYSE: PHG)+
announced today that it introduced the FDA-approved, 160 cm version of its LumiGuide endovascular navigation wire.

Edwards Lifesciences (NYSE: EW)+
announced that it launched its Sapien 3 transcatheter pulmonary valve implant (TPVI) system with Alterra adaptive prestent in Europe.

Medtronic (NYSE: MDT)+
today announced the publication of its novel approach to studying adaptive deep brain stimulation (aDBS).

Commercial partner Ascensia is in discussions with insulin pump manufacturers to create an automated insulin delivery system.