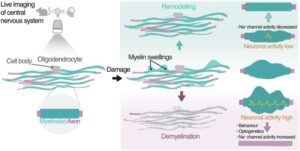
Specialized microscopy enables researchers to visualize the dynamics of myelin swellings
An international research team has gained new insights into the dynamics of myelin swellings in the brain.

An international research team has gained new insights into the dynamics of myelin swellings in the brain.
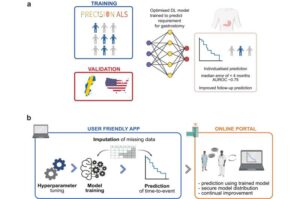
A new AI tool that accurately predicts the need for a feeding tube could transform patient care and improve quality of life for people living with Motor Neuron Disease (MND).
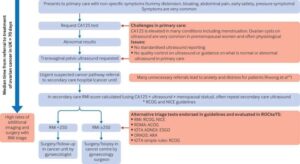
The IOTA ADNEX ultrasound tests picked up 9 out of 10 women with cancer.

For the first time ever, NTNU researchers have identified new characteristics of aggressive prostate cancer.

Researchers led by Min Zhang and Dabao Zhang of the University of California, Irvine’s Joe C. Wen School of Population & Public Health have created the most detailed maps to date showing how genes causally regulate one another across different types of brain cells affected by Alzheimer’s disease.
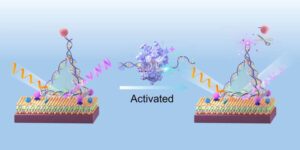
Researchers have developed a highly sensitive light-based sensor that can detect extremely low concentrations of cancer biomarkers in the blood.
Cancer researchers working on immunotherapies have made a big discovery: SLAMF6, a molecule on the surface of immune cells that prevents T cells from effectively attacking tumors—and, in mice, they’ve found a way to neutralize it.
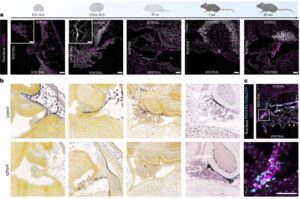
Researchers have identified and characterized a previously unknown cellular barrier in the brain, which sheds new light on how the brain is protected from the rest of the body.

While other drugs clear existing plaques, levetiracetam prevents production of toxic amyloid beta peptides

Driven by overuse and misuse of antibiotics, drug-resistant infections are on the rise, while development of new antibacterial tools has slowed.