Scientists discover eight new schizophrenia genes
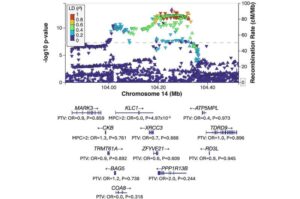
Researchers have discovered eight new genes associated with schizophrenia, in the largest exome-sequencing study of the disorder ever conducted. The breakthrough, made by scientists at the Centre for Neuropsychiatric Genetics and Genomics (CNGG) at Cardiff University, provides new information and improves the understanding and future treatment development for schizophrenia.