BlurryScope: A compact, AI-powered microscope for rapid, cost-effective cancer scoring
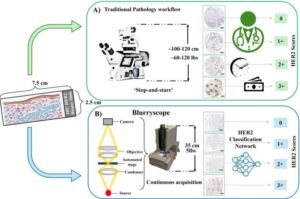
A research team at UCLA, led by Professor Aydogan Ozcan, has introduced BlurryScope, a compact, cost-effective scanning microscope that combines simple optical hardware with advanced deep learning algorithms to assess HER2 status in breast cancer tissue samples.