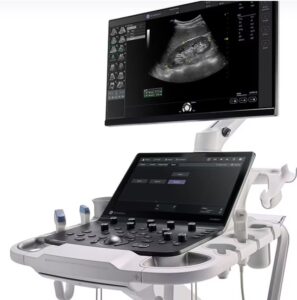
GE HealthCare launches new ultrasound system
GE HealthCare (Nasdaq: GEHC)+
announced that it launched the new Versana Premier, its latest offering from the Versana family of ultrasound systems.

GE HealthCare (Nasdaq: GEHC)+
announced that it launched the new Versana Premier, its latest offering from the Versana family of ultrasound systems.

Promising findings by researchers at Baylor College of Medicine and collaborating institutions could lead to the development of a non-invasive stool test and a new therapy for endometriosis, a painful condition that affects nearly 200 million women worldwide. The study appears in the journal Med.

Medtronic (NYSE: MDT)+ announced today that the FDA approved an early feasibility study to evaluate its Affera system for treating VT.
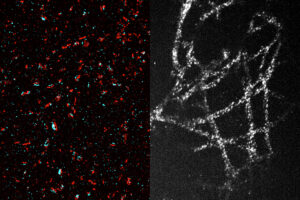
Labs that can’t afford expensive super-resolution microscopes could use a new expansion technique to image nanoscale structures inside cells.

Iota Biosciences, a subsidiary of Astellas Pharma, announced that it received FDA investigational device exemption (IDE) for its bladder implant.

Endostart announced today that it received CE mark approval for the expanded use of its flagship Endorail product.

Johnson & Johnson MedTech (NYSE: JNJ)+
announced today that it launched its VOLT (variable angle optimized locking technology) plating system.

Si-Bone today announced the first patient procedures with its FDA breakthrough device, the iFuse Torq TNT implant system.
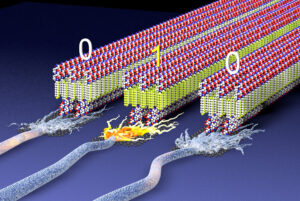
Biodegradable structures could revolutionize energy, information technologies and advanced medicine

AccurKardia announced that it received FDA breakthrough device designation for its aortic valve stenosis (AVS) ECG-based AI screening software.