
InventHelp Inventor Develops Improved Shaver for Surgical Incision Sites (DAL-429)
The invention provides an effective way to shave and remove the hair from a surgical incision site

The invention provides an effective way to shave and remove the hair from a surgical incision site

Medtronic (NYSE: MDT)+
officials say the company received FDA clearance for the next-generation PillCam Genius SB capsule endoscopy kit.
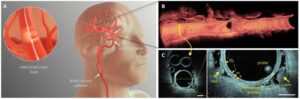
A large international team of micro-engineers, medical technologists, and neurosurgeons, has designed, built and tested a new type of probe that can be used to take pictures from inside arteries in the brain.
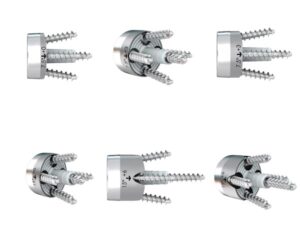
ADDISON, Texas , May 16, 2024 /PRNewswire/ — FX received 510k clearance for its full-wedge augmented glenoid baseplates. The newly cleared baseplates bring 6 new options to the previously cleared portfolio. There are now a combined total of 18 glenoid baseplate options to the market to address a variety of surgeon needs. Augmented glenoid baseplate options have continued to become a growing solution for surgeons to address bone loss, defects, or complicated morphologies of the glenoid.

CARLSBAD, Calif., May 15, 2024 /PRNewswire/ — DCN Dx, a leading contract research organization for in vitro diagnostics, today acknowledges the FDA’s Emergency Use Authorization (EUA) of the WELLlife™ COVID-19/Influenza A&B Test, developed by Wondfo USA. This important authorization will enable healthcare professionals to differentiate rapidly between COVID-19 and influenza infections, enhancing patient care at the point-of-care.
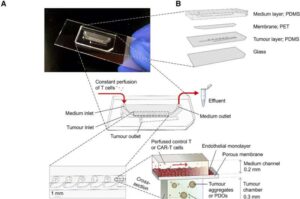
How do tumors react to a certain therapeutic approach? Knowing this before the start of a therapy would be of enormous value for people suffering from cancer as well as for the doctors treating them.

A filter made from yeast encapsulated in hydrogels can quickly absorb lead as water flows through it.

The approval allows women to self-collect vaginal specimens for HPV testing in a health care setting, which could include non-traditional locations such as a retail pharmacy or mobile clinic.
Butterfly Network (NYSE:BFLY) announced today that it launched its first specialty product, the iQ+ Bladder portable ultrasound.
Atraverse Medical announced today that the FDA cleared its Hotwire radiofrequency guidewire left-heart access device.