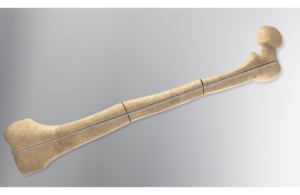
Orthofix wins FDA 510(k) clearance for Rodeo Telescopic Nail
Orthofix (Nasdaq: OFIX)+
today announced it received FDA 510(k) clearance for its Rodeo Telescopic Nail.

Orthofix (Nasdaq: OFIX)+
today announced it received FDA 510(k) clearance for its Rodeo Telescopic Nail.

OrthoXel announced that it received FDA 510(k) clearance for its Vertex hip fracture nail (HFN) for fracture fixation.
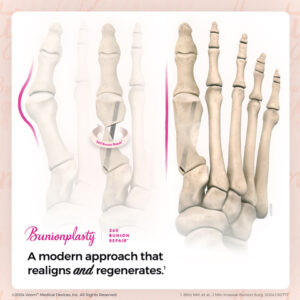
The company is pleased to report a rapidly accelerating interest in its proprietary, minimally invasive Bunionplasty® 360 Bunion Repair™ solution
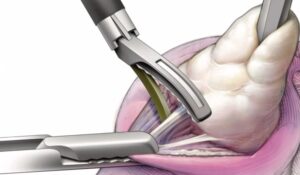
Freyja Healthcare Brings First-of-Its-Kind 2mm Abdominal-Access Device to Laparoscopic Surgery to Further Innovation in Fast-Growing Women’s Health Market
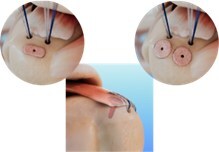
LOS ANGELES, May 8, 2024 /PRNewswire/ — TETROUS, INC., a leading regenerative medicine company today announced that it has completed the first surgical cases using EnFix TAC™, its latest product that expands its line of EnFix™ implants to cover every surgical technique for rotator cuff repair.

4C Medical Technologies announced today that the FDA granted breakthrough device designation for its AltaValve system.

Outset paused shipments of its TabloCart with prefiltration last year after receiving a warning letter from the FDA.

MISGAV, Israel, May 6, 2024 /PRNewswire/ — ZygoFix Ltd. a portfolio company of The Trendlines Group Ltd. (“Trendlines”) announced that it received regulatory clearance from the United States Food and Drug Administration (FDA clearance) for its zLOCK Lumbar Facet Fixation System. This achievement was supported by compelling clinical evidence from the company’s ongoing European clinical study, marking a significant advancement in spinal fusion technology..

In an advancement in gastrointestinal health, researchers from Johns Hopkins University have developed the Multifunctional Ablative Gastrointestinal Imaging Capsule (MAGIC)—a gastrointestinal imaging capsule for esophagus surveillance and interventions.

Scientia Vascular,is thrilled to announce the FDA clearance of two uniquely engineered catheters: the Plato 17 and the Socrates 38.