
Affluent Medical touts first implant of artificial urinary sphincter
Affluent Medical today announced the successful first-in-human implant of its minimally invasive urinary incontinence treatment device.

Affluent Medical today announced the successful first-in-human implant of its minimally invasive urinary incontinence treatment device.

Applied during endoscopic procedures, GastroShield could help prevent complications such as bleeding and leakage from weakened gastrointestinal tissues.

With the new technique, MIT researchers hope to identify mutations that could be targeted with new cancer therapies.
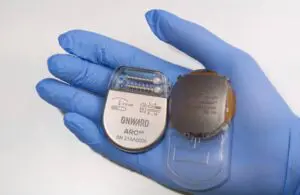
Onward Medical announced today that the FDA accepted it into its new Total Product Lifecycle Advisory Program (TAP) — a move that could boost the development of Onward’s BCI technology.

Pulse Biosciences (Nasdaq:PLSE) announced that the FDA granted 510(k) clearance for its CellFX nsPFA percutaneous electrode system.
One panelist who voted yes said the “incremental benefits outweigh the small risks of anaphylaxis.”

Getinge this week announced a major FDA clearance and the beginning of a U.S. launch of multiple surgical tools.
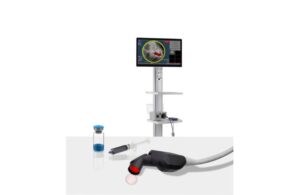
Lumicell announced that an FDA advisory committee voted in support of the benefit-risk profile of its LumiSight offering.

Beckman Coulter Life Sciences, a laboratory automation and innovation company, has received 510(k) clearance from the Food and Drug Administration (FDA) to distribute its DxFLEX Clinical Flow Cytometer in the United States.

SurGenTec announced today that it received FDA 510(k) clearance for its OsteoFlo HydroPutty synthetic bone graft.