
Shape-shifting ultrasound stickers detect post-surgical complications
First-of-its-kind device ‘tags’ an organ to monitor abnormal, life-threatening fluid leaks

First-of-its-kind device ‘tags’ an organ to monitor abnormal, life-threatening fluid leaks

A nanosurgical tool – about 500 times thinner than a human hair – could give insights into cancer treatment resistance that no other technology has been able to do, according to a new study.

The FDA’s decision allows people who don’t take insulin, including those who don’t have diabetes, to use the devices without a prescription.
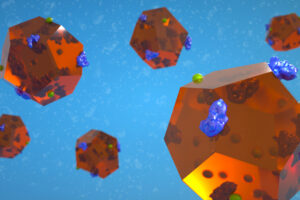
Study shows metal-organic particles can both deliver vaccines and act as an adjuvant to generate a strong immune response at a lower dose.

RDS, the medical device start-up, has been granted the CE mark for MultiSense, its patented, wearable solution for continuous Remote Patient Monitoring.

Dexcom (Nasdaq:DXCM) announced today that the FDA cleared its Stelo glucose biosensor that does not require a prescription.

Biosense Webster, a cardiac arrhythmia treatment specialist and part of Johnson & Johnson MedTech, has received CE mark approval for the VARIPULSE Platform for the treatment of symptomatic drug refractory recurrent paroxysmal atrial fibrillation (AF) using pulsed field ablation (PFA).

Medtronic (NYSE: MDT)+
announced today that it received FDA 510(k) clearance for its OsteoCool 2.0 bone tumor ablation system.
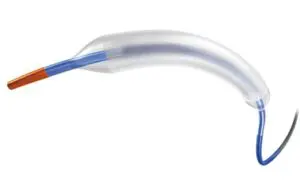
Boston Scientific (NYSE: BSX)+
announced today that it received FDA approval for its Agent drug-coated balloon (DCB).
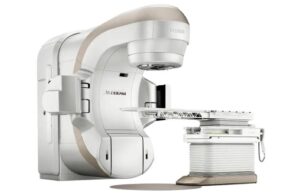
Varian announced today that it received FDA 510(k) clearance for its TrueBeam and Edge radiotherapy systems with HyperSight imaging.