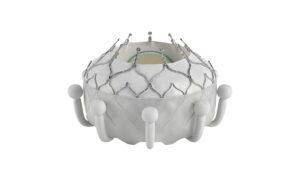
Edwards receives FDA approval for first transcatheter tricuspid valve replacement treatment
The Evoque valve will serve patients with few treatment options, wrote RBC analyst Shagun Singh.

The Evoque valve will serve patients with few treatment options, wrote RBC analyst Shagun Singh.

Fractyl Health — developer of medtech and gene therapies to reverse diabetes and obesity — began trading on the Nasdaq today under the symbol GUTS.
SeaStar Medical Holding Corporation (Nasdaq: ICU) announces the issuance by the Canadian Intellectual Property Office of Canadian Patent No. 2814586
Philips (NYSE: PHG)+
announced today that the FDA granted 510(k) clearance to its latest TEE transducer technology.

InspireMD (Nasdaq:NSPR) announced today that it received CE mark approval for its CGuard embolic prevention carotid stent system (EPS).
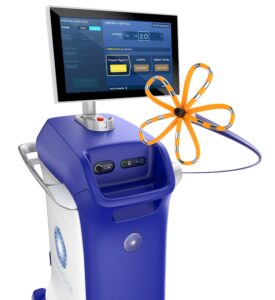
Boston Scientific Corporation (NYSE: BSX) announced it has received U.S. Food and Drug Administration (FDA) approval for the FARAPULSE™ Pulsed Field Ablation (PFA) System. The FARAPULSE PFA System is indicated for the isolation of pulmonary veins in the treatment of drug-refractory, recurrent, symptomatic, paroxysmal (i.e., intermittent) atrial fibrillation (AF) and is a unique new alternative to standard-of-care thermal ablation treatment.

With improvements to the stent graft delivery system enabling a 1 Fr profile reduction on the majority of sizes, the device now offers the most 6 Fr compatible configurations among balloon expandable stent grafts.
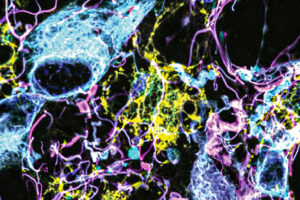
A new microscopy technique that enables high-resolution imaging could one day help doctors diagnose and treat brain tumors.
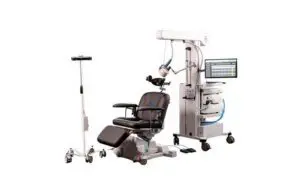
Magstim announced today that it received FDA clearance for its Horizon 3.0 transcranial magnetic stimulation (TMS) system with StimGuide Pro.

A Prospective, Randomized, Multicenter Study Assessing the Safety and Efficacy of the ReCET™ System in Adult Patients with Type 2 Diabetes