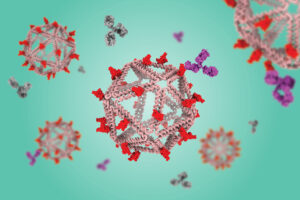
DNA particles that mimic viruses hold promise as vaccines
Using a DNA-based scaffold carrying viral proteins, researchers created a vaccine that provokes a strong antibody response against SARS-CoV-2.

Using a DNA-based scaffold carrying viral proteins, researchers created a vaccine that provokes a strong antibody response against SARS-CoV-2.

Tyber Medical Broadens Its Vast Plating Portfolio with FDA Approval of Additional Screw and Plate Options, and Indications for Mini-Frag System
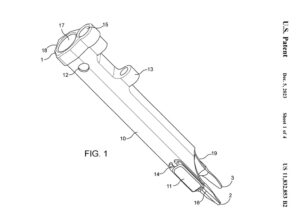
CARLSBAD, Calif., Jan. 29, 2024 (GLOBE NEWSWIRE) — Aurora Spine Corporation (“Aurora Spine” or the “Company”) (TSXV: ASG), a designer and manufacturer of innovative medical devices that improve spinal surgery outcomes, today announced the issuance of its United States Patent No: 11,832,853 entitled “Hybrid Radiolucent Surgical Operating Tube”.

The device is designed to reduce the risk of hernia by distributing suture tension over a large area of tissue.

Element Science announced that it received CE mark approval for its novel patch-wearable cardioverter defibrillator (P-WCD).

The single-use, PEEK-based RIB System utilizes the platform technology of Able’s Valkyrie® Thoracic Fixation System, further strengthening Able Medical’s portfolio.
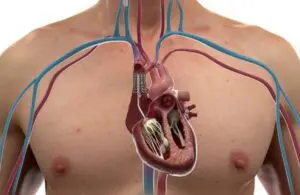
CroíValve announced today that it began an early feasibility study for its Duo tricuspid coaptation valve system.

Abbott (NYSE: ABT)+
announced today that it received approval from the FDA for the launch of its Liberta RC DBS system
Siemens Healthineers announced that the FDA cleared its Syngo Virtual Cockpit, a private, secure communication platform.

Intuitive Surgical (Nasdaq: ISRG)+
announced today that it received CE mark approval for its da Vinci SP surgical robot platform.