Imvaria and Darmiyan receive FDA authorizations for AI-enabled diagnostic tools
The de novo classifications cover software for detecting a lung condition and assessing dementia risk.

The de novo classifications cover software for detecting a lung condition and assessing dementia risk.

NeuroSigma today announced that the U.S. Food and Drug Administration (FDA) cleared use of NeuroSigma’s second-generation Monarch eTNS System for treating pediatric attention deficit hyperactivity disorder (ADHD)
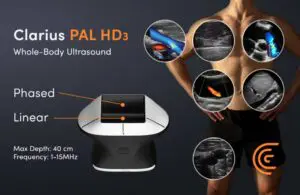
Clarius Mobile Health announced today that it received CE mark approval for its wireless handheld whole-body ultrasound scanner.

DermaSensor announced today that the FDA cleared its real-time, non-invasive, AI-powered skin cancer evaluation system.
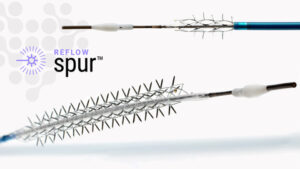
Reflow Medical, Inc., a developer of innovative medical devices focused on cardiovascular disease, announces it has received CE (Conformité Européenne) Mark certification in the European Union for the Bare Temporary Spur Stent System.
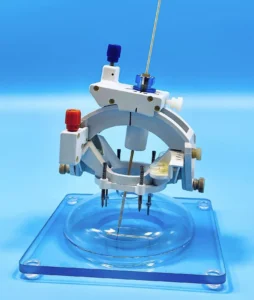
Limited Market Release of First Purpose-Built OR Product to Begin in First Half of 2024
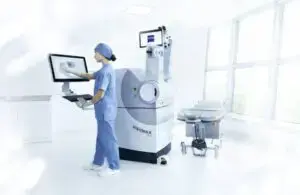
Zeiss Medical Technology announced that the FDA approved the VisuMax 800 with Smile Pro software.
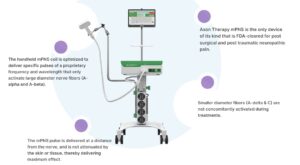
Neuralace Medical announced today that it received FDA clearance for its chronic painful diabetic neuropathy (PDN) treatment.

Zeta Surgical announced today that the FDA granted it a special 510(k) clearance for expanded software functionality.

3Spine’s MOTUS device is a ‘first-of-kind’ breakthrough medical technology indicated for the biomechanical reconstruction and stabilization of a spinal motion segment