
NuEyes wins FDA approval for augmented reality surgical visualization tech
NuEyes announced today that the FDA granted approval to its patent-pending NuLoupes augmented reality smart glasses technology.

NuEyes announced today that the FDA granted approval to its patent-pending NuLoupes augmented reality smart glasses technology.

Glaukos (NYSE:GKOS) announced today that the FDA approved a New Drug Application (NDA) for its iDose TR implant.
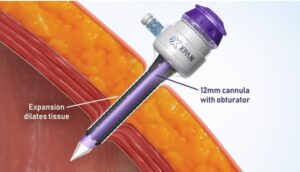
The XpanTM Universal Trocar System is a first of its kind, radially expanding trocar that can be tailored by surgeons during laparoscopic procedures to accommodate 3 mm up to 12 mm instruments
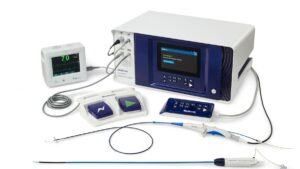
The FDA has approved the Medtronic (NYSE: MDT)+
PulseSelect pulsed field ablation (PFA) system, the device developer said today.
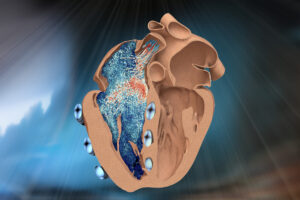
The realistic model could aid the development of better heart implants and shed light on understudied heart disorders.
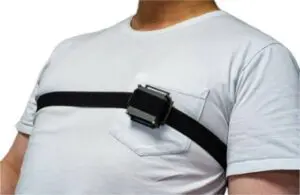
Neuranics announced today that it plans to launch a magnetic sensor development kit for monitoring the magnetic activity of the heart.

Medtronic has received CE mark for its Percept RC neurostimulator for deep brain stimulation (DBS). The smallest and thinnest device on the market, Percept RC is the a rechargeable DBS system with BrainSense sensing technology.

Omini, a medtech company developing the first multiplexed blood testing platform for personalised medicine and chronic disease management, has been given approval by European authorities of a patent for its multi-gate Organic Electro-Chemical Transistor (OECT)-based detection device comprising depolarisation electrode.
The clearance positions BD and partner Babson Diagnostics to support blood collection from community sites such as pharmacies.
CAMH-led pre-clinical studies using a small molecule drug have shown promise as a potential new treatment for multiple sclerosis (MS). The results have been published in the journal Science Advances.