
FDA approves next-gen intrathecal drug delivery system from Medtronic
Medtronic (NYSE:MDT) announced that the FDA approved its next-generation SynchroMed III intrathecal drug delivery system

Medtronic (NYSE:MDT) announced that the FDA approved its next-generation SynchroMed III intrathecal drug delivery system
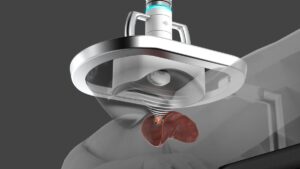
The system offers a minimally invasive alternative to surgical resection and thermal ablation in patients with primary and metastatic liver cancers.

FluidAI’s first solution, Stream™ Platform, uses advanced sensors and an AI-driven algorithm that may prompt surgeons to identify these leaks earlier, helping surgeons achieve better clinical outcomes and efficiencies within the greater healthcare system.

ZKR Orthopedics, Inc., a clinical stage medical device company, today announced that its LIFT implant technology has been granted Breakthrough Device Designation by the U.S. Food and Drug Administration (FDA).
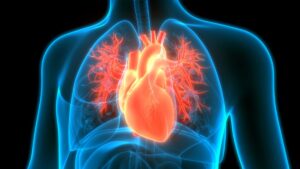
Patients who received catheter ablation had a 57% lower risk of developing heart failure than their counterparts on antiarrhythmic drugs.

24-month data shows UI symptom relief is durable, and early data shows symptom relief for pelvic organ prolapse
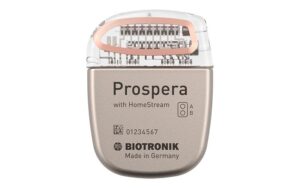
Biotronik today announced positive results from a clinical trial for its Neuro Prospera spinal cord stimulation (SCS) system.

Large study finds three genes strongly linked to vegetarianism

Participants who screened positive for autism had a nearly 41% probability of being diagnosed with the condition.
The money also will help fund clinical trials for additional indications and the expansion of its manufacturing capabilities.