MedTech News
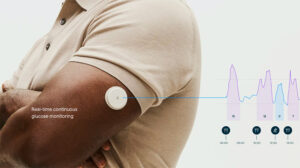
Abbott wins FDA clearance for duo of over-the-counter CGMs
Abbott (NYSE: ABT)+ today announced FDA clearance for two over-the-counter continuous glucose monitor (CGM) systems, Lingo and Libre Rio.
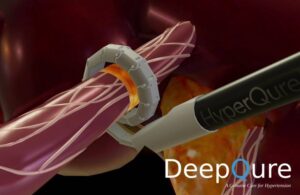
DeepQure wins FDA IDE nod for renal denervation study
DeepQure announced today that it received FDA investigational device exemption (IDE) approval for a study of its HyperQure system.

CroíValve reports positive first-in-human valve repair data
CroíValve today announced favorable patient outcomes in a first-in-human clinical trial of its Duo tricuspid coaptation valve system.

J&J’s DePuy Synthes wins new FDA clearance for Velys surgical robot
Johnson & Johnson MedTech‘s DePuy Synthes announced today that it received a new FDA 510(k) clearance for its Velys surgical robot platform.

FDA clears portable blood, IV fluid warmer from Mequ
Mequ announced today that the FDA granted 510(k) clearance to its M Warmer system, a portable blood and IV fluid warmer platform.
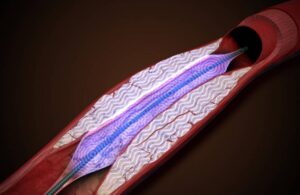
Amplitude Vascular Systems wins FDA IDE approval for intravascular lithotripsy study
Amplitude Vascular Systems announced today that it received FDA investigational device exemption (IDE) for its pulsatile intravascular lithotripsy (PIVL) therapy.

Abbott wins CE mark for dual-chamber leadless pacemaker
Abbott (NYSE: ABT)+ announced today that it received CE mark approval for its Aveir dual-chamber (DR) leadless pacemaker system.
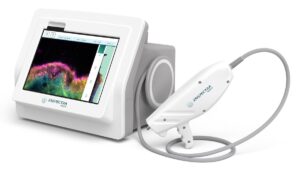
Enspectra Health Receives FDA Breakthrough Device Designation for AI-powered Imaging Platform Targeting Skin Cancer
Enspectra Health, a health tech company, today announced that the U.S. Food and Drug Administration (FDA) has granted Breakthrough Device Designation for its next generation AI-powered VIO™ Skin Platform (VIO)
