MedTech News

Absolutions receives FDA breakthrough designation for abdominal wall closure device
The device is designed to reduce the risk of hernia by distributing suture tension over a large area of tissue.

Element Science wins CE mark for patch-wearable cardioverter defibrillator
Element Science announced that it received CE mark approval for its novel patch-wearable cardioverter defibrillator (P-WCD).

Able Medical Announces 510(k) Clearance of New RIB System Indicated for Thoracic Repair
The single-use, PEEK-based RIB System utilizes the platform technology of Able’s Valkyrie® Thoracic Fixation System, further strengthening Able Medical’s portfolio.
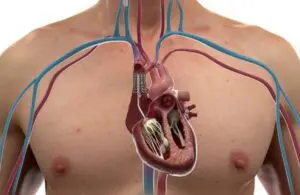
CroíValve wins FDA IDE for tricuspid heart valve
CroíValve announced today that it began an early feasibility study for its Duo tricuspid coaptation valve system.

Abbott wins FDA nod for world’s smallest rechargeable DBS system with remote programming
Abbott (NYSE: ABT)+
announced today that it received approval from the FDA for the launch of its Liberta RC DBS system
FDA clears Syngo Virtual Cockpit tech from Siemens Healthineers
Siemens Healthineers announced that the FDA cleared its Syngo Virtual Cockpit, a private, secure communication platform.

Intuitive wins CE mark for da Vinci SP surgical robot
Intuitive Surgical (Nasdaq: ISRG)+
announced today that it received CE mark approval for its da Vinci SP surgical robot platform.

Sequana Medical announces DSMB approval to start randomized MOJAVE cohort – US Phase 1/2a study of DSR® 2.0 for treatment of heart failure
DSMBi rates DSR 2.0 as safe following review of data from non-randomized cohort
Data from non-randomized cohort confirms dramatic improvement in diuretic response and at least 95% reduction in loop diuretic requirements up to almost four months after last DSR therapy
First patient enrolled in randomized controlled cohort expected in Q1 2024
