MedTech News
.................... by Andrew Celentano

Naox Technologies wins FDA clearance for earbud-based EEG
Naox Technologies has received FDA 501(k) clearance for its wired electroencephelography (EEG) earphones, Naox Link.
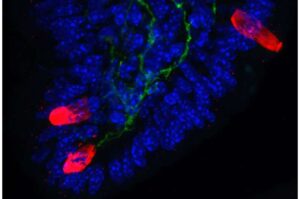
Pain-sensing neurons kick-start immune responses that drive allergies and asthma
Pain-sensing neurons in the gut kindle inflammatory immune responses that cause allergies and asthma, according to a new study by Weill Cornell Medicine.
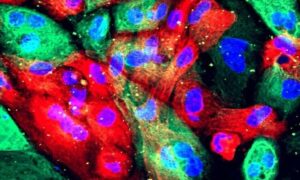
Study links dozens of blood proteins to prostate cancer risk across populations
A large-scale study has identified dozens of blood proteins linked to prostate cancer risk, some shared across populations, some unique to specific groups.

Self-guided behavioral app helps children with epilepsy sleep earlier
An evidence-based web-app helped children with epilepsy to fall asleep on average 16.5 minutes earlier.
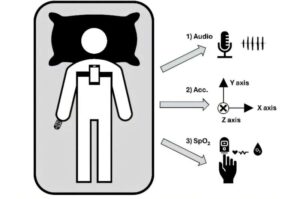
Mobile technology improves sleep apnea diagnosis after a stroke
Sleep apnea is a disorder characterized by repeated obstructions or collapses of the upper airway during sleep. These interruptions to breathing reduce the amount of oxygen in the blood. Stroke patients are at high risk, as they experience poorer sleep quality and have a higher prevalence of sleep disorders, such as insomnia and respiratory problems.
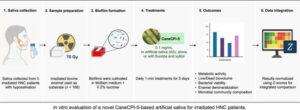
Artificial saliva with sugarcane protein shields teeth after cancer treatment
An artificial saliva in the form of a mouthwash, produced with the CANECPI-5 protein extracted from sugarcane and modified in a laboratory, can aid in treating teeth in patients with head and neck cancer. In these cases, radiotherapy very close to the mouth can destroy salivary glands and compromise saliva production, which is essential for controlling bacteria and disease.
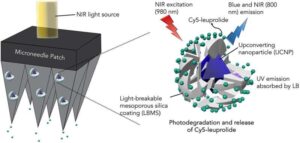
Light-triggered microneedle patch could make IVF hormone delivery painless and automated
A McGill University research team has developed a painless, automated way to deliver in vitro fertilization (IVF) hormones using a light-activated microneedle patch, an innovation that could ease one of the most stressful parts of fertility treatment and open new possibilities for other diseases that require frequent, time-sensitive injections.
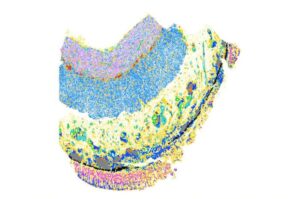
Researchers uncover molecular roots of tissue scarring in inflammatory bowel disease
In earlier work, a team led by researchers at the Broad Institute and Mass General Brigham discovered a key cell type underlying fibrosis in inflammatory bowel disease (IBD). Now, in a new study published in Nature, the team has characterized the crosstalk between this and other types of cells that leads to fibrosis.
