MedTech News
.................... by Andrew Celentano

Novel prosthetic design combines AI and 3D printing to improve fit
A new, fully customizable 3D printed socket design is set to transform the prosthetics industry.
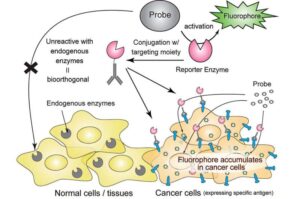
Precision tumor imaging with a fluorescence probe and engineered enzymes—selective activation enables easy visibility
In tests on mice, we delivered a special enzyme to tumors and used a fluorescence probe that only turns on when that enzyme is present,

Engineered immune cells show promise against brain metastases in preclinical study
A new study by researchers at Wake Forest University School of Medicine has identified a promising strategy to treat brain metastases, one of the most challenging and deadly complications of lung cancer.

Smart patch detects allergies before symptoms strike
A wearable device that alerts people with food allergies before a reaction begins has the potential to reduce life-threatening anaphylaxis and transform allergy management from reactive to preventive care.
A molecular ‘switch’ could make pancreatic cancer more treatable
Scientists at Duke-NUS Medical School have identified a molecular “switch” that determines whether pancreatic cancer cells resist chemotherapy or respond to it—a finding that could help convert some of the most treatment-resistant tumors into forms that are more manageable with existing drugs.

Painless skin patch offers new way to monitor immune health
Researchers at The Jackson Laboratory (JAX), in collaboration with the Massachusetts Institute of Technology (MIT), have developed the first bandage-like microneedle patch that can sample the body’s immune responses painlessly from the skin.

Abbott wins FDA approval for updated heart failure monitoring device
The company redesigned the reader to fit in a carry-on suitcase and be easier to use day to day.

Novocure has reimbursement win for Optune Lua in Japan
Novocure (Nasdaq:NVCR) announced today that Japan’s Ministry of Health, Labour and Welfare (MHLW) approved reimbursement for its Optune Lua system.
