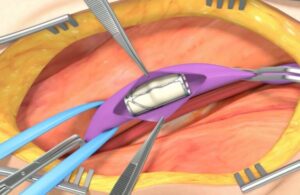
EnVVeno has first-in-human heart valve data, expects FDA decision this year
EnVVeno Medical (Nasdaq:NVNO) announced three-year outcomes for its heart valve that it aims to submit to the FDA this year.

EnVVeno Medical (Nasdaq:NVNO) announced three-year outcomes for its heart valve that it aims to submit to the FDA this year.

Revolutionary system combines ultrasonics and ultraviolet energy to destroy airborne pathogens and pollutants beyond traditional purification limits.
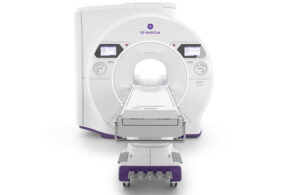
GE HealthCare (Nasdaq: GEHC)+
today unveiled Signa Sprint, an ultra-premium wide-bore 1.5% high-performance gradient MRI system.

Launch of Zoom DuoPort enables Continuous Dual Aspiration Technique (CDAT) for safer, faster procedures
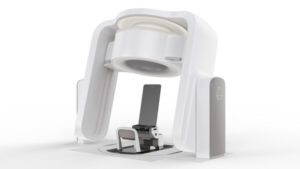
MADISON, Wis., May 6, 2025 /PRNewswire/ — The Food and Drug Administration Agency (FDA) has updated the regulatory status of Marie®, Leo Cancer Care’s upright particle therapy solution, to pending. Marie combines Leo Cancer Care’s upright patient positioning system with their upright fan beam CT scanner and can be utilized with any fixed particle beam.
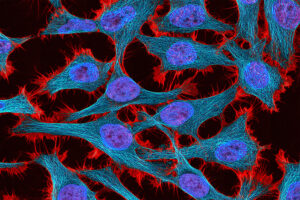
The circuits could help researchers develop new treatments for fragile X syndrome and other diseases caused by mutations of a single gene.
Innovative mesh aims to improve patient outcomes by naturally absorbing into the body post-surgery.

Novocure (Baar, Switzerland) announced today that it has won a CE mark for its Optune Lua wearable device for treating metastatic non-small cell lung cancer.

ICU Medical (Nasdaq: ICUI) announced today that it received FDA 510(k) clearance for several new additions to its infusion pump portfolio.

Resmed (NYSE: RMD)+
announced today that it launched its NightOwl home sleep apnea test across the U.S.