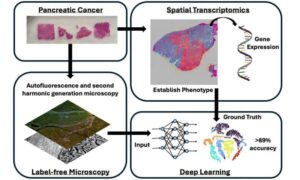
AI-based tool can ‘measure’ cancer aggressiveness and paves the way for new therapies
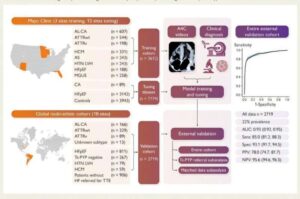
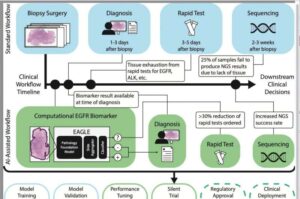
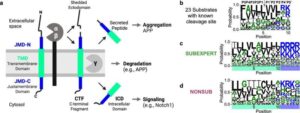
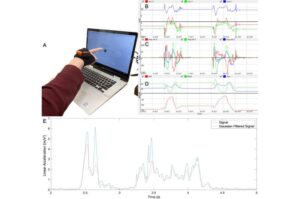
As cancer cases have increased worldwide, the disease has become more complex, presenting challenges to scientific advances in diagnosis and treatment. In this context, artificial intelligence (AI) has emerged as a valuable tool for predicting and detecting cases.