Scientists develop novel self-healing electronic skin for health monitoring
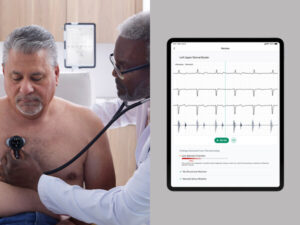
Researchers have achieved a breakthrough in wearable health technology by developing a novel self-healing electronic skin (E-Skin) that repairs itself in seconds after damage. This could potentially transform the landscape of personal health monitoring.