FDA clears AI-powered breast cancer detection tech from iCad
iCad (Nasdaq:ICAD) announced today that the FDA cleared its ProFound Detection version 4.0 for digital breast tomosynthesis (DBT).

iCad (Nasdaq:ICAD) announced today that the FDA cleared its ProFound Detection version 4.0 for digital breast tomosynthesis (DBT).
LEUVEN, Belgium, Oct. 1, 2024 /PRNewswire/ — Relu, a pioneer in artificial intelligence (AI) assisted segmentation for dental labs and software companies, proudly announces the dual achievement of 510(k) clearance by the U.S. Food and Drug Administration (FDA) and CE Mark approval by an EU Notified Body. These regulatory milestones authorize the commercial distribution of the Relu® Creator, the cutting-edge dental tool that enables users to create 3D anatomical models from patients in just minutes.

Nevro (NYSE:NVRO) announced today that it received FDA approval for an AI-powered, personalized pain management platform for spinal cord stimulation.

CENTER VALLEY, Pa., Sept. 5, 2024 /PRNewswire/ — Odin Medical Ltd., an Olympus Corporation company, has received U.S. Food and Drug Administration (FDA) 510(k) clearance for the first cloud-based Artificial Intelligence (AI) technology designed to assist gastroenterologists in detecting suspected colorectal polyps during colonoscopy procedures, the CADDIE™ computer-aided detection (CADe) device.
PARIS, Sept. 3, 2024 /PRNewswire/ — European MedTech startup AZmed, recognized as a leading company in AI for medical imaging, today announced that its Rayvolve solution has received 510(k) clearance from the Food and Drug Administration (FDA) for detecting fractures on pediatric X-rays. This milestone comes two years after receiving FDA clearance for adult fracture detection, positioning Rayvolve as a top-performing solution for detecting fractures in adult and pediatric patients. The clearance was supported by an independent study conducted with SimonMed Imaging, one of the largest outpatient imaging providers in the U.S.

Coronary artery disease (CAD) is the most common cause of illness-based death throughout the world. According to the World Health Organization, CAD causes 17.9 million deaths per year worldwide, nearly one-third of all illness-based deaths annually.
The comprehensive cloud-based platform combines AI diagnostics and detailed measurements for rapid and accurate diagnosis of structural heart disease and heart failure.
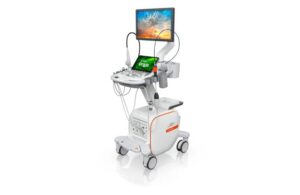
Siemens Healthineers announced today that it received FDA clearance for its Acuson Origin ultrasound system and AcuNav Lumos 4D ICE catheter.
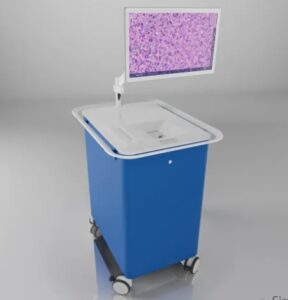
SANTA CLARA, Calif., Aug. 9, 2024 /PRNewswire/ — Invenio Imaging, a leader in intraoperative fresh tissue imaging and artificial intelligence (AI), announced today the enrollment of the first patients in a US pivotal study of its AI-based image analysis module for lung cancer

MIDDLETON, Wis., Aug. 7, 2024 /PRNewswire/ — Natus Medical Incorporated has announced the launch of autoSCORE, the first-of-its-kind artificial intelligence model capable of automatic and comprehensive clinical EEG interpretation, providing accuracy on par with medical experts.